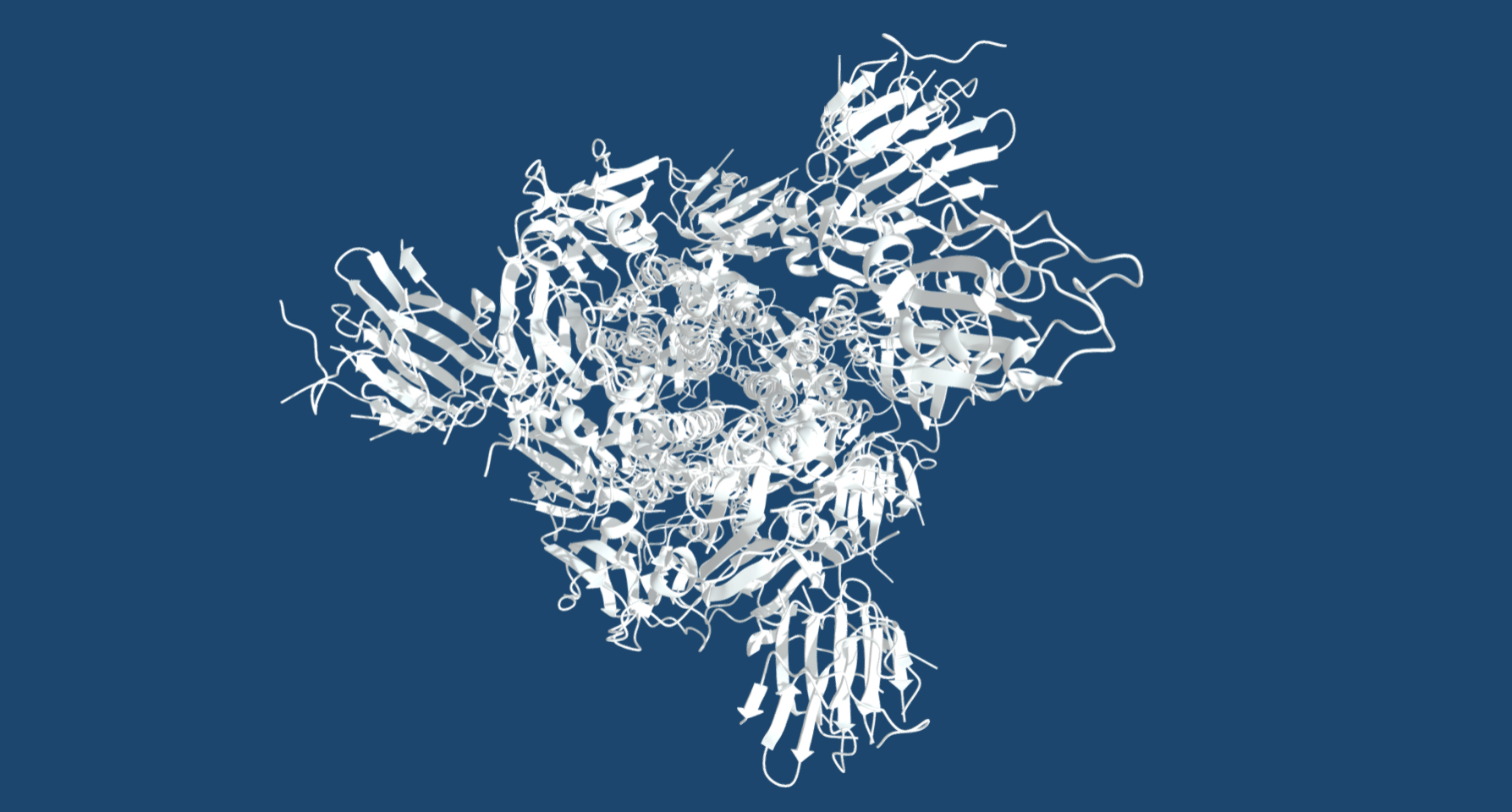
A team of scientists has created a three-dimensional map showing how a small molecule with anticancer properties – called spliceostatin – can promote the killing of cancer cells. This fundamental discovery could ultimately help researchers design future cancer drugs.
The team, from The Institute of Cancer Research, London, revealed how spliceostatin binds the spliceosome – a very large molecular machine that rearranges genetic information within cells through a process known as splicing. While disruption of splicing can lead to cancer, modulation of splicing with spliceostatin can have antitumour effects.
Spliceostatins are representative for a larger class of compounds that have emerged as promising cancer treatment agents due to their ability to target cancer cells. Some of these molecules have even entered clinical trials for various types of cancers.
Mapping out the details
Although spliceostatin was discovered 25 years ago, it has proved challenging to visualise in complex with spliceosomes due to technical limitations.
Recent technological advances in cryo-electron microscopy – a Nobel Prize-winning technique that involves freezing proteins and blasting them with electrons to produce images of individual molecules – have made it possible for researchers to capture the precise molecular details and reconstruct spliceostatin’s 3D shape.
Dr Constantin Cretu, a postdoctoral researcher from the Mechanisms and Regulation of Pre-mRNA Splicing Team led by Professor Vlad Pena, has established the isolation of a human spliceosome complex arrested with spliceostatin.
Using a combination of X-ray crystallography and cryo-electron microscopy, he obtained 3D structures of spliceostatin and sudemycin (another small molecule with anticancer properties) compounds bound to spliceosomal complexes.
Spliceostatin is a powerful tool for dissecting the dynamics of spliceosomes in cells because it can ‘freeze’ the macromolecular machine in its natural state. By capturing the 3D structure of these spliceosomes, the researchers could visualise and understand spliceostatin’s effects on the splicing process in unprecedented detail.
